Sample Preparation for XRF Analysis
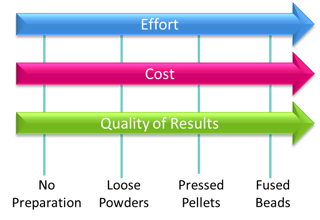
XRF( X-ray Fluorescence Spectrometry) is a comparative chemical analysis technique that is capable of analyzing a wide range of materials in different forms for a large part of the periodic table. This versatility makes it applicable to a wide range of applications from quality control for metal alloys, to the analysis of sulfur in gasoline to heavy metals in plastics and electronics. XRF can analyze almost any material you can present to the spectrometer, but the better you prepare a sample the more accurate your analytical results. Your choice of sample preparation will always be a balance of the quality of results your require, the effort your are willing to expend ( labor, complexity) and the cost( sample preparation equipment, labor, time to analysis). Your choice may be different for different materials depending on your analysis requirements.
How do you choose what sample preparation method is best for your application?
Here we review the 5 most common ways to prepare samples for XRF analysis and what you need to consider with each method.
Solid Samples
Solid samples can be anything from unprepared pieces of metal or electronics or plastics to cut and polished metal samples. The ideal sample for XRF analysis will have a perfectly flat surface. Irregular sample surfaces change the distance from the sample to the x-ray source and introduce error. All XRF systems are calibrated based on a fixed sample to source distance. Changing the distance can increase or decrease the intensity coming from any element contained in the sample.
Solid samples such as metal alloys, can be analyzed with no sample preparation or they can be cut and polished for a more quantitative analysis. This topic is explored in our blog post How to Prepare Metal Alloy samples for Analysis by XRF.

Even for largely flat samples, surface finish can affect your analysis, particularly for lighter elements. Rough surfaces can cause scattering and re-absorption of longer wavelength elements. This effect is energy dependent so while the Ni signal may not be affected, the signal from C or S could be dramatically reduced. Quantitative analysis of solid samples often requires finishing the surface with a lathe or grinding paper. The finer the finish the better the results will be for the lightest elements.
testing. It is also important to note that the sample preparation you choose should be applied to your calibration standards as well as any unknown samples.
Loose Powders
The analysis of loose powdered material usually requires that the sample be placed into a plastic sample cup with a plastic support film. This insures a flat surface to the X-ray analyzer and the sample to be supported over the X-ray beam. The more finely ground the sample the more likely it is to be homogeneous and have limited void spaces providing for a better analysis. Sufficient powder should be used to insure infinite thickness is obtained for all of the elements of interest. This requirement can be met by using 15g of sample for most materials. Special care should be taken for the analysis of metal powders in high power (2-4Kw) WDXRF instruments. The sample can heat up during analysis and melt through the support film resulting in abrasive powder being spilled directly into your instrument.
Pressed Pellets
Pressing powder into pellets is a more rigorous sample preparation than pouring loose powders into a sample cup. The process includes grinding a sample into a fine powder, ideally to a grain size of <75um, mixing it with a binding /grinding aid and then pressing the mixture in a die at between 20 and 30T to produce a homogeneous sample pellet. The binding /grinding aid is usually a cellulose wax mixture and combines with the sample in a proportion of 20%-30% binder to sample.
This sample preparation approach provides better analytical results than loose powders because the grinding and compression creates a more homogeneous representation of the sample with no void spaces and little sample dilution. This leads to higher intensities for most elements than loose powders. Pressed pellets are still susceptible to particle size effects if not ground fine enough, but the biggest limitation to this approach is the mineralogical effects which most predominantly affect the analysis of major elements. Pressed pellets are excellent for the analysis of elements in the ppm range. Pressed pellets are also relatively simple and inexpensive to prepare only requiring a pulverizing mill and sample press.
Fused Beads
Sample prepared as fused beads provide a near perfectly homogeneous representation of the sample to the XRF and is considered by many to be the ideal sample preparation method for solids. Fused beads are created by mixing a finely powdered (<75um) sample with a flux in a flux/sample ratio of 5:1 to 10:1 and then heated to 900C-1000C in a platinum crucible. The sample is dissolved in the flux(usually a lithium tetraborate or tetraborate/metaborate mixture )and cast into a mold with a flat bottom. The resultant glass disc or fused bead is a homogeneous representation of the sample free of mineral structures. The  benefits of this approach are the reduction of mineralogical or matrix effects leading to more accurate analyses and the ability to combine several different matrix types into the same calibration curve. Downsides include the relatively high sample dilution which has a negative effect on the analysis of trace elements and the higher cost associated with this type of sample preparation (fusion equipment, platinum crucibles and consumables) Typical fused beads are also only approximately 3mm thick and thus are susceptible to infinite thickness issues for heavier elements. Fused beads usually require higher initial costs between platinumware and a fusion device, but then have similar cost/sample to prepare as pressed pellets.
benefits of this approach are the reduction of mineralogical or matrix effects leading to more accurate analyses and the ability to combine several different matrix types into the same calibration curve. Downsides include the relatively high sample dilution which has a negative effect on the analysis of trace elements and the higher cost associated with this type of sample preparation (fusion equipment, platinum crucibles and consumables) Typical fused beads are also only approximately 3mm thick and thus are susceptible to infinite thickness issues for heavier elements. Fused beads usually require higher initial costs between platinumware and a fusion device, but then have similar cost/sample to prepare as pressed pellets.
Liquids
Liquids are prepared by pouring them into a plastic sample cup in the same way as loose powdered samples. There are limited options for analyzing liquid samples and the main trick is to choose the correct support film that provides a balance of strength and transmission capabilities and contamination. Mylar is a good general purpose film often used for the analysis of sulfur in fuels or lubricating oils. Polypropylene has better transmission than Mylar but has a lower tensile strength. Kapton is the "bomb proof” film but dramatically attenuates your signal for lighter elements and is susceptible to strongly basic solutions. If you are going to analyze liquids you will need to do a little research into selecting the best support film for your analysis goals. If you are using your XRF to analyze sulfur in fuels, you should read our article entitled "What you need to know about the analysis of S in fuels by WDXRF" for more detailed information on this applciation.
Conclusion
There are many ways to prepare samples for XRF analysis and the method you choose will be a balance of the sample type, the amount of effort you are willing to expend and the quality of results you require.
Have questions about XRF analysis ? Need help choosing the right sample preparation method or reference materials ? Our team of specialists can assist you. Just ask an expert !



